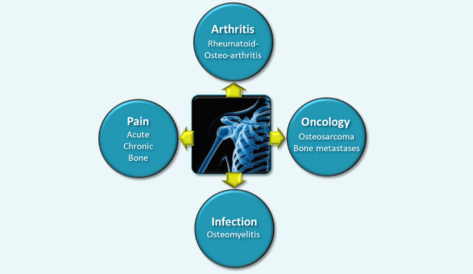
Our pipeline
CANDIDATE: 12b80 / ODD status granted by EU
PHASE: end regulatory pre-clinical/CMC
INDICATION: Osteosarcoma (first step) and extend to bone metastases
NEXT STEPS: Clinical phase in humans.
CANDIDATE: 18A184
PHASE: POC in two moderate/severe rat models of OA
INDICATION: acute/severe and chronic osteoarthritis
NEXT STEPS: CMC and Pre-clinical development
CANDIDATE: Vectorized-VX-765
PHASE: early research
INDICATION: metastatic breast cancer
NEXT STEPS: POC in rodents models
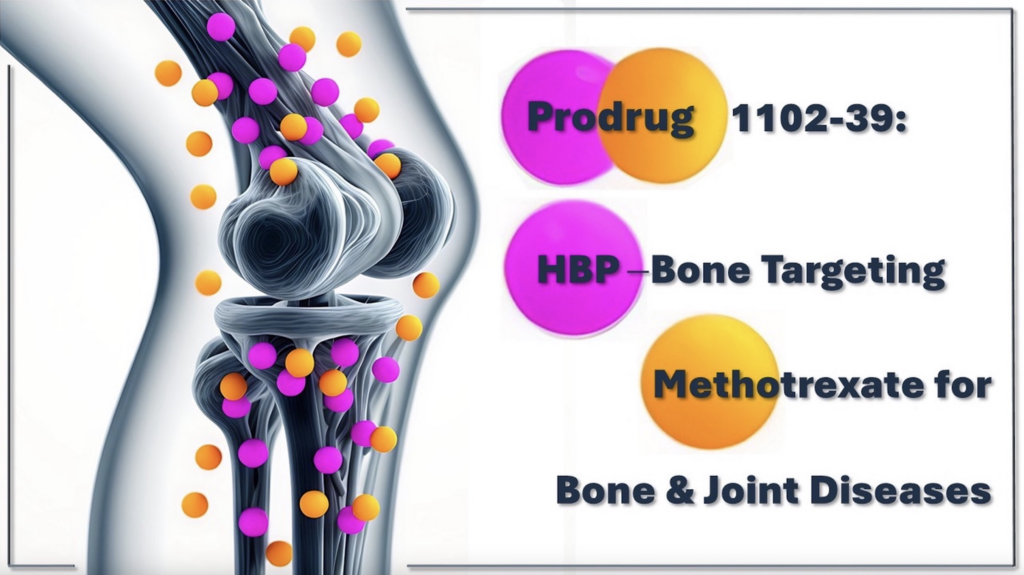
Selective bone targeting, local drug release and improved therapeutic index.
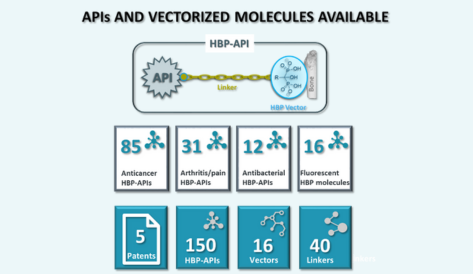
Active Pharmaceutical Ingredient (API) – Vectorization of molecules with different functional groups – NCEs
Custom linkers
– Selective local drugs release (pH, enzymes, …)
– Modulation of drug’s LogP, solubility, transport, …
Innovative Bone Targeting Vectors
– Improved drug distribution Vectors
– Modulation of retention in the bone
– Modulation of antiresorptive action
API releasing group
– Key for API liberation
– Modulates API release rate and cleavage conditions
Engineering innovative HBP vectors for targeting bones and joints
Last news
- October 2024
 October 2024 – Atlanthera announces the publication
October 2024 – Atlanthera announces the publication - June 2024
 Atlanthera announces a research collaboration with the University of Sheffield to find new therapy against breast tumours and breast derived bone metastasis …
Atlanthera announces a research collaboration with the University of Sheffield to find new therapy against breast tumours and breast derived bone metastasis … - March 2024
 At the occasion of the World Rare Disease Day , our team wants to make a meaningful impact on osteosarcoma and on those affected by rare diseases.
At the occasion of the World Rare Disease Day , our team wants to make a meaningful impact on osteosarcoma and on those affected by rare diseases.
Contact our Team
« * » indique les champs nécessaires